Bomb Radiocarbon and Age Validation
Despite the global acceptance of otolith annuli as the best means for estimating the age of most fish species, the correct interpretation of the annuli is far from trivial, and can result in serious and systematic ageing error. Indeed, aside from the use of tagged, hatchery-reared fish released into the wild, confirming the accuracy of a method of annulus interpretation for marine fish species is often problematic. Mark-recapture of chemically-tagged individuals has generally been considered to be the most accurate means of confirming the frequency of formation of presumed annuli, through comparison of time at liberty with the number of annuli deposited distal to the chemical check. While the approach is sound, extremely low recapture rates for fish at liberty more than 2-3 years can make it difficult to acquire sufficient samples for an adequate test. Moreover, the technique validates the time elapsed since tagging, not the absolute age of the fish. Alternatively, radiochemical dating based on 210Pb : 226Ra or 228Th : 228Ra ratios can be used to differentiate between very different age interpretations, but these assays are too imprecise for detailed or individual age confirmations. The most widely used approach, that of the seasonal progression of marginal increments, is well suited only to fast-growing fish, and suffers from the lack of an objective means of evaluation. Thus there is a well defined gap in our ability to confirm the age interpretations of the majority of marine fish species, particularly those that are long-lived. However, the recent finding that nuclear testing left a dated mark in the otolith provides a significant breakthrough in our ability to determine accurate, absolute ages for individual long-lived fish.
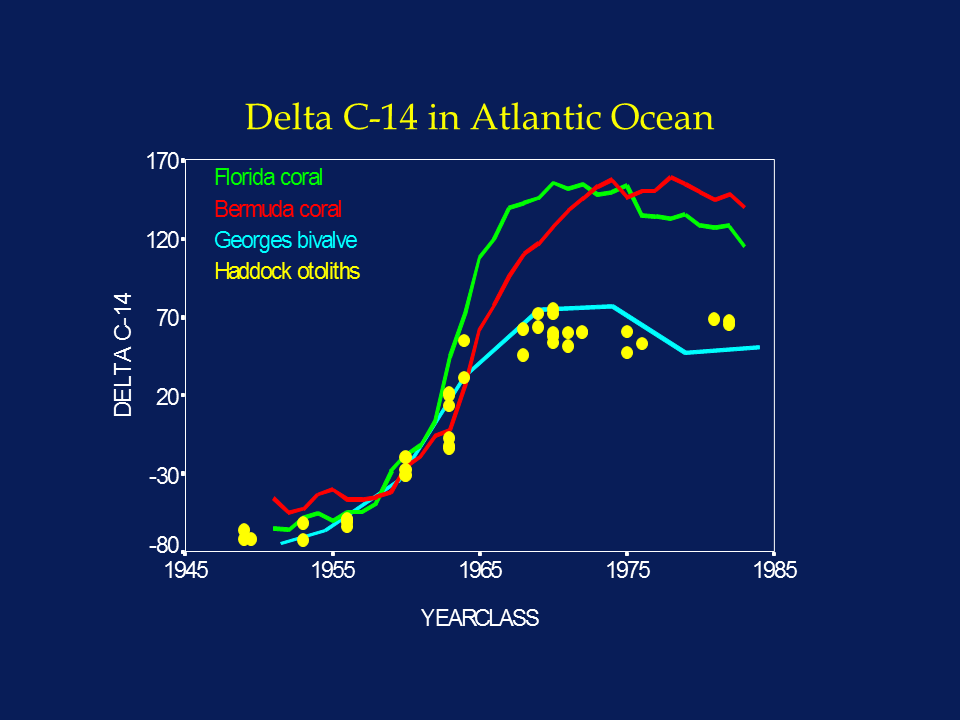
The widespread atmospheric testing of atomic bombs in the 1950's and 1960's produced a 100% increase in atmospheric radiocarbon, which was quickly incorporated into the world's oceans. Analysis of annular growth rings in coral demonstrated that bomb radiocarbon was incorporated into the accreting coralline structure in concentrations proportional to those present in the water column. Thus the time series of bomb radiocarbon recorded in the coral was shown to reflect that present in the marine environment, which increased by about 20% between 1950 and 1970. Using accelerator mass spectrometry (AMS) as a sensitive and accurate assay tool, Kalish (1993) was able to demonstrate that the otoliths of a New Zealand fish species also incorporated 14C, and that the time series of radiocarbon reconstructed from the presumed otolith annuli was similar to that present in nearby corals. Thus he was able to infer that the otolith annuli had been interpreted and aged correctly, because systematic under- or over-ageing would have resulted in a phase shift between the otolith 14C and the coral 14C time series.
Subsequent work by both Kalish and our laboratory has confirmed the value of the bomb radiocarbon technique for solving problems of age validation in a variety of fish species. Furthermore, recent work in our laboratory has confirmed that the uptake of 14C in young fish otoliths is synchronous with that of both corals and bivalves in the North Atlantic. Such large-scale synchronicity implies that the 14C time series reconstructed from the otolith cores of old fish can be compared to one of the other North Atlantic time series; errors in annulus-based age determinations would manifest themselves as non-coherent time series.
In light of the sharp rate of increase of the 14C signal associated with the onset of nuclear testing, interpretation of the 14C chronology in otolith cores is relatively simple; the otolith chronology should match other published chronologies for the region as long as the annular age assignments (= year-class) are correct. Any under-ageing would phase shift the otolith 14C chronology towards more recent years, while over-ageing would phase shift it towards earlier years. Because marine waters with D14C values greater than 00/00 did not generally exist prior to the late 1950's, coastal fish otolith cores with sub-zero values must have formed before the late 1950's. Even contamination with material of more recent origin could only increase the 14C value, not decrease it. Thus the 14C value sets a minimum age to the sample, and the years 1958-1965 become the most sensitive years for 14C-based ageing.
While techniques such as the mark-recapture of chemically-tagged fish can be used to accurately validate the annual frequency of formation of growth increments in the otolith, especially in young or abundant fishes, only radiocarbon from nuclear testing has the potential to confirm both annulus formation and absolute age in individual fish. All studies to date suggest that bomb radiocarbon can be used to confirm the accuracy of an ageing method to within 1-3 yr, or even less than 1 year in special circumstances (Melvin and Campana 2010).
The only constraints to this procedure are the relatively high cost (~$700-$1000 per otolith) and the requirement for fish hatched during the 1958-65 period, so as to take advantage of the unique 14C values during that period. While the availability of suitable otolith samples may limit the applicability of this approach to specific stocks and species, use of bomb-derived radiocarbon as a dated otolith marker appears to provide one of the most accurate and logistically feasible methods for the age validation of long-lived species that is currently available.
For further information and the results of recent studies, see Campana (1997), Campana and Jones (1998), Campana et al. 2008, Neilson and Campana (2008), Bruch et al. (2009), Davis-Foust et al. (2009), Francis et al. (2010), Armsworthy and Campana (2010) and Melvin and Campana (2010). A review of the field is presented in Campana (1999). The Methods page provides details of otolith preparation protocols.