Trancriptional co-option, transcriptional decay and the principles of regulatory evolution.
Arnar Pálsson, Institute of biology, University of Iceland
Marcos A. Antezana, Institute of biology, University of Iceland
PALSSON A. & ANTEZANA M. 2017: Transcriptional co-option, transcriptional decay and the principles of regulatory evolution. Abstract. - In: W ERTH S. & O BERMAYER W. (editors). Lichen Genomics Workshop II. Institute of Plant Sciences, University of Graz, Austria. 2–5 November 2017. - Fritschiana (Graz) 85: 32–34. - ISSN 1024-0306.
Regulatory evolution is important for adaptive evolution and the emergence of novelties, in part because regulatory mutations tend to be less pleiotropic than changes in exons. The functional properties of regulatory elements, e.g. short, degenerate motifs, for multiple activators and repressors, dictate the evolution of regulatory DNA (Wray et al. 2003). Stabilizing selection on transcription may explain the evolutionary turnover of transcription factor binding sites (TFBS) in enhancers (Ludwig et al. 2000). Genes have been recruited for multiple functions during evolution, by a process called transcriptional co-option (TC) (True and Carroll 2002). In TC a mutation in a gene (here called focal gene), not previously expressed in a specific tissue or cell population during development, turns the gene on in that tissue and the responsible allele is fixed in the population. As new TFBS arise easily via mutation, it was proposed that any transcription factor can co-opt (influence the transcription of) any gene in the genome (Prud’homme et al 2007). Here we define the opposite scenario, evolution by transcriptional decay (TD). In TD, a mutation reducing strongly the transcription of a focal gene in tissue or developmentally specific fashion is fixed by positive selection. An example of TC would be the recruitment of various crystallins to the vertebrate lens (Piatigorsky 2006), and of TD the loss of Pitx1 expression in pelvic structures in stickleback (Shapiro et al. 2004). Here we will use transcriptional decay and co-option as a prism to explore the principles of regulatory evolution.
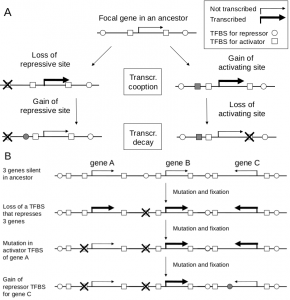 TC or TD can arise by mutations in either trans-factors (TF) or cis-elements (figure part A). While we focus on mutations in cis, changes in the structure or tissue specific concentration of a TF can affect expression of a focal gene. Such changes are expected to be pleiotropic, with changes in expression of targets of that TF (if changes are in TF level, then the effects will be more circumscribed). Changes at the level of cis-elements will be less pleiotropic. Disruption of TFBS can for example either increase or decrease expression of the focal gene, depending on whether the TF that binds that TFBS represses or activates transcription. The complementary argument applies for mutations that generate TFBS; TC can occur by gain of an activator binding site affecting the focal gene. And in TD a gain of a binding site for repressor near the focal gene.
TC or TD can arise by mutations in either trans-factors (TF) or cis-elements (figure part A). While we focus on mutations in cis, changes in the structure or tissue specific concentration of a TF can affect expression of a focal gene. Such changes are expected to be pleiotropic, with changes in expression of targets of that TF (if changes are in TF level, then the effects will be more circumscribed). Changes at the level of cis-elements will be less pleiotropic. Disruption of TFBS can for example either increase or decrease expression of the focal gene, depending on whether the TF that binds that TFBS represses or activates transcription. The complementary argument applies for mutations that generate TFBS; TC can occur by gain of an activator binding site affecting the focal gene. And in TD a gain of a binding site for repressor near the focal gene.
Regulatory mutations can be pleiotropic. In one scenario, a mutation alters the expression of two or more genes in the same chromosome region. If the fitness increase of the increased expression of the focal gene outweighs the fitness reduction of its chromosome neighbors (figure, part B), then TC can occur. The stronger the selection, the more serious the regulatory side effects can be. After fixation of such a mutation, we anticipate rounds of refinement where the deleterious expression of nearby genes will be alleviated, e.g. by loss of activator sites or gain of repressor sites (figure, part B). Other regulatory changes (miRNA binding sites, RNA degradation etc) may play a role, and the complementary case when reduced expression of two or more genes is favored will adhere to the same principles. Similar mechanistic and evolutionary logic will also be apply if adaptive expression increase of a focal gene in a tissue, leads to increased expression of the gene in other tissues.
The central principles discussed here are, i) mutations disrupting TFBS can lead to beneficial changes in gene expression, ii) cis-regulatory mutations can be pleiotropic, affecting multiple genes and potentially tissues, iii) the likelihood of fixation of such pleiotropic mutations depends on the strength of selection, iv) natural selection is likely to alleviate such side-effects by favoring modifiers (e.g. in cis or trans). Finally, these principles are likely to apply more generally, to gain and decay of associations between trans-regulators and regulatory motifs in DNA, RNA and proteins. For instance regulatory systems like those controlling mRNA splicing, export, stability and localization, translation, protein maturation and modifications and other cellular regulatory cascades.
Acknowledgements. We thank Silke Werth and other organizers of the Lichen Genomics Workshop.
References
Wray G.A., Hahn M.W., Abouheif E., Balhoff J.P., Pizer M., Rockman M.V., Romano L.A. 2003: The evolution of transcriptional regulation in eukaryotes. Molecular Biology and Evolution.20: 1377-1419.
Ludwig M.Z., Bergman C., Patel N.H., Kreitman M. 2000: Evidence for stabilizing selection in a eukaryotic enhancer element. Nature. 403: 564-567.
True J.R., Carroll S.B. 2002 Gene co-option in physiological and morphological evolution. Annual Review Cellular and Developmental Biology. 18: 53-80.
Prud'homme B., Gompel N., Carroll S.B. 2007 Emerging principles of regulatory evolution. Proceedings of the National Academy of Sciences U S A. 15: 8605-8612.
Piatigorsky J. 2006 Evolutionary genetics: seeing the light: the role of inherited developmental cascades in the origins of vertebrate lenses and their crystallins. Heredity. 96: 275-277.
Shapiro M.D., Marks M.E., Peichel C.L., Blackman B.K., Nereng K.S., Jónsson B., Schluter D., Kingsley D.M. 2004 Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature. 428: 717-723
