Catalysis
Catalysis is an important key enabling technology that supports our economies because the world’s most successful industrial sectors such as petroleum, chemicals production, energy generation, food production etc. rely heavily on catalysis. Improvements in the efficiency and cost effectiveness of separation technologies and catalysis have significant potential impact on global energy consumption. We are interested in developing heterogeneous catalyst based on MOFs and incorporating catalytic sites (transition metal and rhodium/ruthenium) onto solid supports.
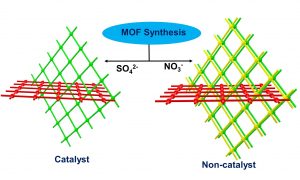
We have synthesized catalytically active metal–organic frameworks (MOFs) with copper(II) paddle-wheel clusters and urea–carboxylate linker, which was achieved at room temperature in the presence of sulfate anions. The role of various anions in determining the MOF structure was analyzed using X-ray diffraction. Structural analysis of the MOFs indicated that a two-fold interpenetrated rhombus grid (HI-101) was formed in the presence of sulfate anions, but a three-fold interpenetrated square grid network (HI-102) was obtained with nitrate and perchlorate anions. HI-101 proved to be an efficient catalyst for the cycloaddition of CO2 at room temperature, the oxidation of primary alcohols to aldehydes and the methanolysis of epoxides, but the other MOFs were not catalytically active. Dalton Trans, 2022, 51, 16316-16324