Information from this page should be cited as: Campana, S.E. 2001. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 59:197-242.
Age determination is invariably accompanied by various sources of error. A variety of methods exist through which age interpretations can be validated. The methods described below are the best available for insuring ageing accuracy, whether in support of large-scale production ageing or a small-scale research project.
Click here to see some features, advantages and disadvantages of methods used to confirm the accuracy of age interpretations. Methods are listed in descending order of scientific value. Growth structure refers to either annulus (A) or daily growth increment (D), depending on application. Methods for age corroboration (such as length frequency analysis and tag-recapture analysis) are not shown here, but are described in detail in Campana (2001).
Release of Known Age and Marked Fish into the Wild
Release of known age and marked fish into the wild is probably the most rigorous of the age validation methods for many species, since the absolute age of the recaptured fish is known without error. Since the released fish are generally less than 1 yr old, recaptured fish will have spent the majority of their lives in natural surroundings. Fish can be marked either externally, as in the case of salmon with coded wire tags (Quinn et al., 1991), or immersion mass-marked using temperature fluctuations (Volk et al., 1999) or chemicals (Campana, 1999) so as to leave a permanent mark on the bony structures used for ageing. This approach is not well suited to long-lived species, since recapture rates of old fish tend to be minimal. Nor can this method be used on species which cannot be reared in captivity prior to release. Nevertheless, this method has been used with success to confirm absolute age and growth increment formation at both the daily (Tsukamoto & Kajihara, 1987; Secor et al., 1995b) and the yearly scale (Fitzgerald et al., 1997; Svedang et al., 1998).
There are two variations on this method which make it more widely available at the expense of relatively minor assumptions. The first variation involves scale removal at the time of tagging and release of wild fish. Where tagging has been restricted to relatively young fish, and where scale annuli have been found to be reliable indicators of age at that young age, the removed scale can be used to estimate the age at tagging, and subsequently be added to the time at liberty to estimate the absolute age of the fish. Where the age at tagging is short compared to the time at liberty, the advantage of this approach is that the wild tagged fish effectively become known age at release and thus need not be reared in captivity (e.g. Matlock et al., 1993). A second variation on this theme involves the tagging of young fish where age can reasonably be approximated by size. This approach was used by Lee et al. (1995) in their study of bluefin tuna (Thunnus thynnus L.), whereby tuna estimated to be 1-3 years old at the time of tagging were subsequently recaptured up to 15 years later. Although there was a ±1 year margin of error around the age estimate at the time of tagging, that margin was too small to change the conclusion that vertebral growth marks were formed annually after tagging.
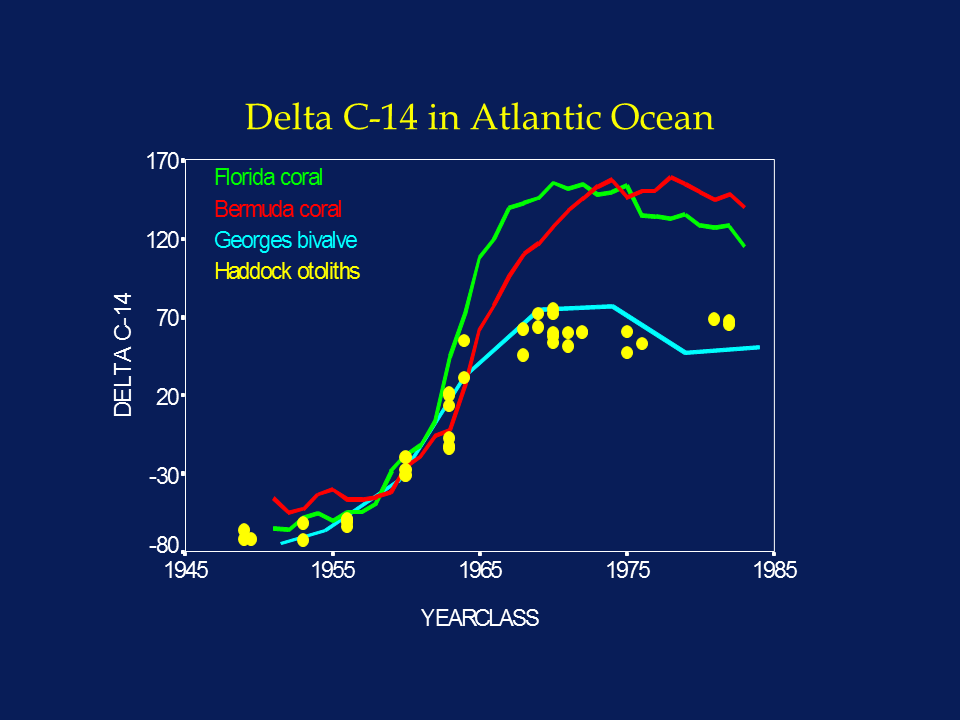
Bomb Radiocarbon
Bomb radiocarbon derived from nuclear testing provides one of the best age validation approaches available for long-lived fishes (Kalish, 1993, 1995a,b; Kalish et al., 1996, 1997; Campana, 1997, 1999; Campana & Jones, 1998; Campana et al. 2008; Neilson and Campana 2008). The onset of nuclear testing in the late 1950s resulted in an abrupt increase in atmospheric 14C, which was soon incorporated into corals, bivalves, fish and other organisms that were growing at the time. Thus the period is analogous to a large-scale chemical tagging experiment, wherein all otolith cores of fish hatched before 1958 contain relatively little 14C and all those hatched after 1968 contain elevated levels. Fish born in the transition period contain intermediate levels. As a result, the interpretation of the 14C chronology in a sample of otolith cores is relatively simple; the otolith-based 14C chronology spanning the 1960s should match other published 14C chronologies for the region (whether from otoliths or other calcified organisms) as long as the annular age assignments (= year-class) are correct. Any under-ageing would phase shift the otolith 14C chronology towards more recent years, while over-ageing would phase shift it towards earlier years. Otolith contamination with material of more recent origin can only increase the D 14C value, not decrease it. Thus the otolith D 14C value sets a minimum age to the sample, and the years 1958-1965 become the most sensitive years for D 14C-based ageing. For fish born during this time period, bomb radiocarbon can be used to confirm the accuracy of more traditional ageing approaches with an accuracy of at least ± 1-3 yr; the discriminatory power of samples born before or after this period is more than an order of magnitude lower. Since the 14C signal recorded in deepsea and freshwater environments is different from that of surface marine waters (deepsea=delayed; fresh water=advanced), reference 14C chronologies appropriate to the environment experienced during the period of otolith core formation must be used (Kalish, 1995b; Campana & Jones, 1998). Clearly, this approach is not well suited to studies of short-lived (< 5 yr) species, in instances where the presumed hatch dates do not span the 1960s, or in environments where appropriate reference chronologies are not available. On the other hand, the low radioactive decay rate of 14C implies that both archived and recent collections are appropriate for assay.
A more complete description of bomb radiocarbon-based age validation is available on the Bomb Radiocarbon section of this site. Francis et al. (2010) published a statistical method for testing the precision of bomb radiocarbon-based age validations.
Mark-Recapture of Chemically-Tagged Wild Fish
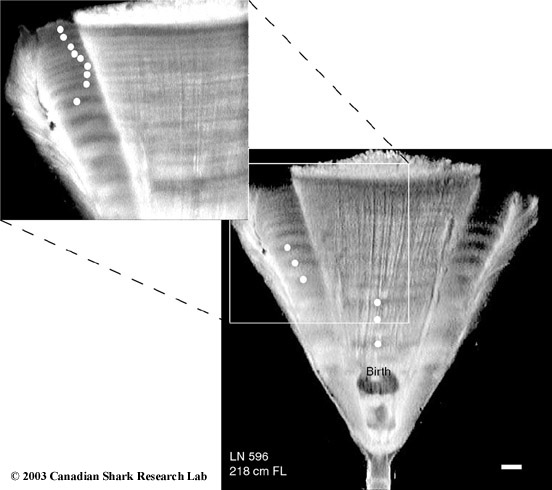
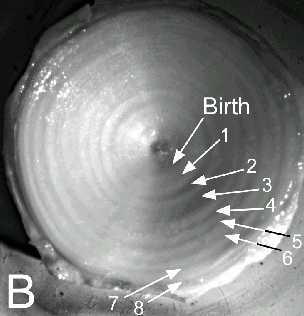
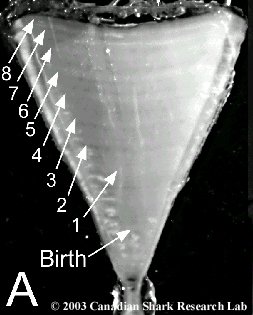
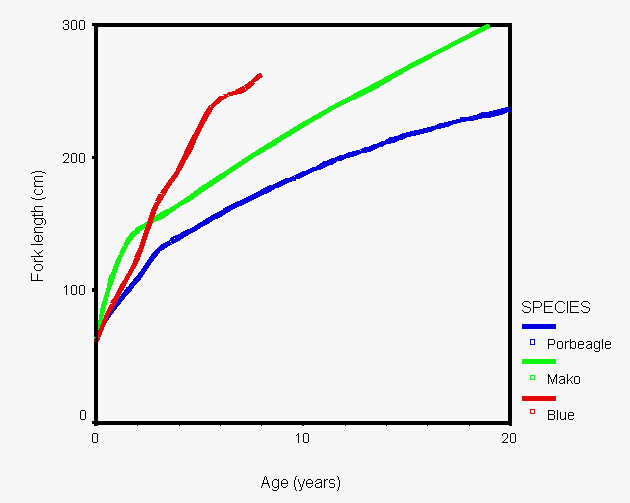
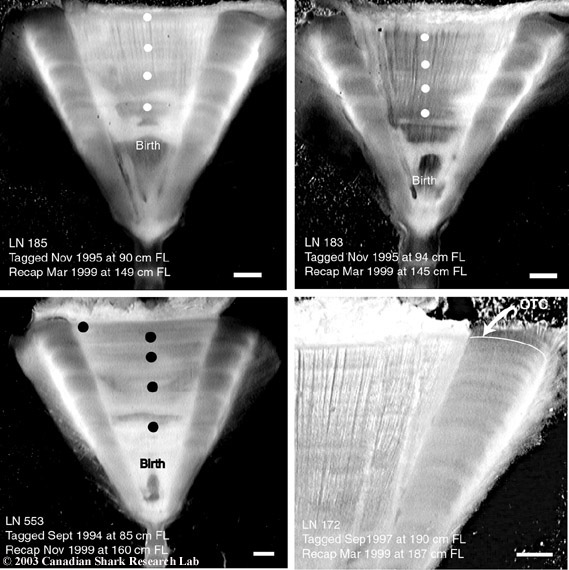
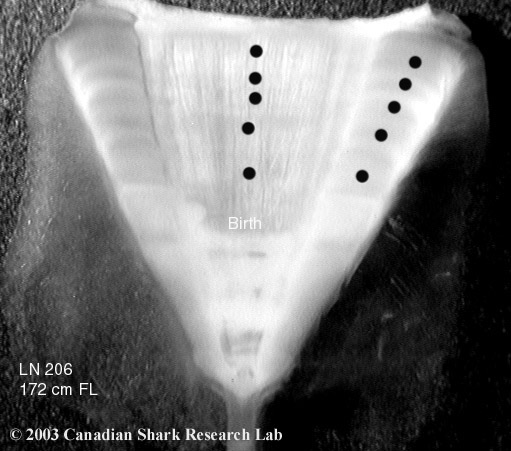
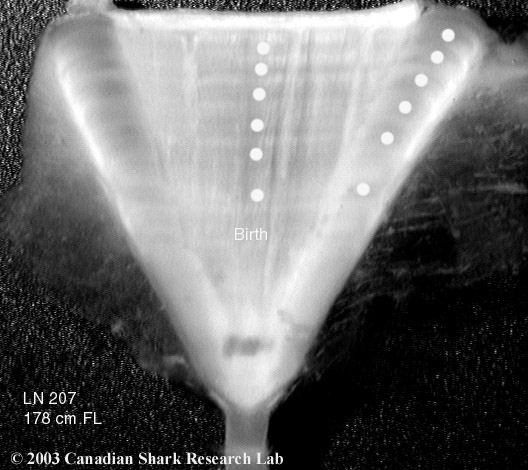
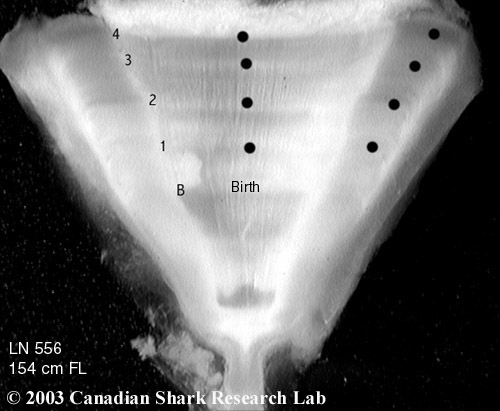
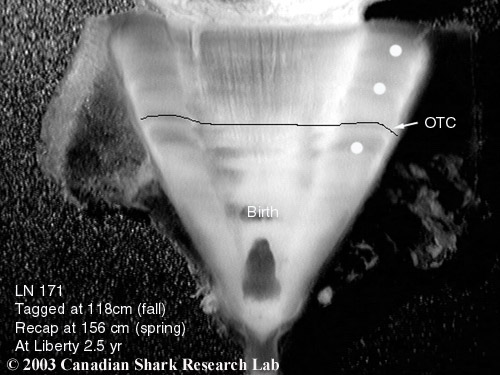
Mark-recapture of chemically-tagged wild fish is one of the best methods available for validating the periodicity of growth increment formation. The method is based on rapid incorporation of calcium-binding chemicals such as oxytetracyline, alizarin, calcein or strontium, applied at the time of tagging, into bones, scales, spines and otoliths (Campana, 1999). Application is through immersion, injection or feeding, although injection is the most practical method for tagging studies of wild fish (Geffen, 1982; Foreman, 1987; Francis et al., 1992; Oliveira, 1996). The result is a permanent mark, visible under fluorescent light (except strontium), in the growth increment being formed at the time of tagging. The number of growth increments formed distal to the chemical mark is then compared to the time at liberty after tagging. This approach has been used to validate annulus formation in a wide variety of structures and species, including sablefish otoliths (Beamish & Chilton, 1982), shark vertebrae (Brown & Gruber, 1988), pike cleithra (Casselman, 1974), spiny dogfish spines (Beamish & McFarlane, 1985), and coral reef fish otoliths (Fowler, 1990). The approach has also been used successfully at the microstructural level, validating daily increment formation in a variety of tuna species (Wild & Foreman, 1980; Laurs et al., 1985). A major advantage of this approach is that the growth increments being validated are formed while the fish is growing in a natural environment. Experiments in which fish are chemically-tagged and then reared in the laboratory or an outside enclosure (Campana & Neilson, 1982; Schmitt, 1984) are less optimal, although they are logistically easier to carry out. A disadvantage of the chemical tagging approach is that the number of increments formed after tagging is often low, resulting in a potentially large relative error if one of the increments (such as that at the growing edge) is misinterpreted. For example, misinterpretation of a single growth zone in a fish at liberty 2 years would result in a 50% error, whereas the same misinterpretation in a fish at liberty 10 years would only produce a 10% error. This effect was highlighted in a recent study in which long-term mark-recaptures detected problems with annulus identification that were not evident from short-term recaptures in the same study (Beamish & McFarlane, 2000). For this reason, fish tagged at a young age and recaptured at an old age provide the most robust validation results (Natanson et al., 2001). Notwithstanding the caveat that this method only validates growth increment formation for the size/age of fish tagged, this is a powerful method, and one of the few readily applied to adult wild fishes.
Radiochemical Dating
Radiochemical dating of otoliths is based on the radioactive decay of naturally occurring radioisotopes which are incorporated into the otolith during its growth. Once incorporated into the otolith, the radioisotopes decay into radioactive daughter products, which are themselves retained within the acellular crystalline structure. Since the half-lives of the parent and daughter isotopes are known (and fixed), the ratio between them is an index of elapsed time since incorporation of the parent isotope into the otolith. By restricting the assay to the extracted otolith core (as opposed to the whole otolith), objective, accurate estimates of absolute age are possible (Bennett et al., 1982; Campana et al., 1990, 1993; Fenton et al., 1990, 1991; Smith et al., 1991; Kastelle et al., 1994; Milton et al., 1995; Burton et al., 1999; Campana, 1999). The isotopic concentrations requiring measurement are exceedingly low, resulting in assay precisions which are often less than optimal, although recent methodological changes have substantially improved precision (Andrews et al., 1999). Current discriminatory power is on the order of 5 yr for 210Pb:226Ra and 1-2 yr for 228Th:228Ra, over age ranges of 0-40 and 0-8 yr, respectively. Therefore, this approach is best suited to long-lived species where the candidate age interpretations are widely divergent, such as in Sebastes or Hoplostethus (Campana et al., 1990; Fenton et al., 1991).
Progression of Discrete Length Modes Sampled for Age Structures
Progression of discrete length modes sampled for age structures has seldom been applied rigorously, but it is a reasonably robust approach for validating the interpretation of annuli in young fish. By monitoring the progression of discrete length modes across months within a year, it is relatively straight forward to determine if the modes correspond to age classes (Natanson et al. 2001). In instances where the length modes are well separated, can be tracked throughout the year, are not confounded by size-selective mortality, migration or multiple recruitment pulses within a year, and the mode corresponding to the young-of-the-year can be unequivocally identified, absolute age is confirmed. Examination of the ageing structures sampled from those same modes can then be used to test the validity of the presumed annuli as age indicators. This was the basis of the approach by Hanchet & Uozumi (1996), who found good correspondence between the number of presumed annuli and the age of the first three well-defined length modes (where the age was confirmed by modal progression). This approach is not equivalent to that which is more commonly applied, in which discrete length modes observed in a single sample are each assumed to correspond to an age class (Shirvell, 1981; Morales-Nin, 1989). While such an approach provides corroboration for an age interpretation, there is no independent evidence that the length modes represent age classes; thus strictly speaking, an approach that does not track modal progression through the year does not validate either absolute age or annulus periodicity. In principle, sampled modal progression should also be applicable to daily age validation. In practice however, size-selective mortality and/or migration is often pronounced in young fishes, thus invalidating the assumption that a distinct cohort is being tracked (Meekan & Fortier, 1996).
Capture of Wild Fish with Natural, Date-Specific Markers
Capture of wild fish with natural, date-specific markers is an approach that has many of the same advantages and disadvantages of bomb radiocarbon dating, since it relies on a large-scale event that applies a dated mark to all fish in a population. In the specific (and rare) instances in which it can be applied, this method can be used to validate growth increment formation over a substantial portion of a fish's life history. For example, both Blacker (1974) and Rauck (1974) reported the presence of otolith annuli which appeared to be characteristic of specific year-classes, such as the characteristically narrow 2nd year growth zone of one year-class of Bear Island cod. More recently, MacLellan & Saunders (1995) suggested that the El Nino-induced disruption of growth in one year-class of Pacific hake (Merluccius productus Ayres) could be used as a dated marker to validate the frequency of annulus formation in fish from this year-class as it grew older. Ogle et al. (1994) further developed the approach in what they termed 'temporal signature analysis', calibrating against distinct increment patterns common to most fish. In general however, such natural marks would seldom be expected to be unambiguously identifiable in individual fish, and in any event, would have to be monitored over a number of years to insure that the mark remained identifiable.
A related but different approach is to take advantage of physiologically-generated marks or checks on the ageing structure, such as the hatching, emergence or 1st feeding check of salmonids (Marshall & Parker, 1982). This can be a powerful validation method of either absolute age or increment periodicity, as long as the date of check formation can be determined through independent observation, and as long as the identity of the check is unambiguous. In the salmonid example above, once an observer had noted the date of emergence of a specific fish from the gravel, the emergence check had become a dated mark on the otolith of that fish that could then be used to validate both the absolute age and the frequency of formation of the daily increments formed until the date of capture. This method is probably better suited to daily increment validation than to annulus validation, since hatch checks (Campana & Neilson, 1985) and settlement marks (Wilson & McCormick, 1997) are common in some groups of fishes. Nevertheless, analogous marks do exist in many older fish, such as the otolith transition zone associated with the onset of sexual maturity (Francis & Horn, 1997). In all cases however, a key requirement is the independent observation of the date of the physiological event, since without it, the check is associated with an age, but not a date of formation.
Marginal Increment Analysis
Marginal increment analysis (MIA) is the most commonly used, and the most likely to be abused, of the validation methods. The underlying premise as a method for validating increment periodicity is sound: if a growth increment is formed on a yearly (daily) cycle, the average state of completion of the outermost increment should display a yearly (daily) sinusoidal cycle when plotted against season (time of day) (e.g. Hyndes et al., 1992; Fowler & Short, 1998; Morales-Nin et al., 1998; Carlson et al., 1999). The popularity of this method can be attributed to its modest sampling requirements and low cost. However, in many ways, this is one of the most difficult validation methods to carry out properly, due to the technical difficulties associated with viewing a partial increment affected by variable light refraction through an edge which becomes increasingly thin as the margin is approached, as well as light reflection off the curved surface of the edge. The absence of an objective means of interpreting the data further complicates the situation. In their review of annulus seasonality studies, Beckman & Wilson (1995) interpreted the results of 104 MIA studies, concluding that about 30% of the species from a given region formed annuli at times different than that of the other species. It is possible that annuli did not form in all of these species, or that the time of opaque zone formation varied widely among species. Indeed, Beckman & Wilson (1995) highlighted the current lack of understanding of the mechanisms underlying annulus formation. However, a more likely explanation is that the MIA technique itself was of low resolving power. Even more problematic are studies which attempt to validate daily increment formation with MIA, working near the resolution limit of light, and confounded by the presence of subdaily increments. Although daily MIA based on transmission electron microscopy (Zhang & Runham, 1992) or using otoliths with unusually broad increments (Jenkins & Davis, 1990) has some merit, MIA studies of daily increments are, in general, of questionable value.
Marginal increment analysis is sometimes differentiated from edge analysis, but when used as a validation method, has similar properties. The marginal increment is usually calculated as a proportional state of completion, ranging from near zero (an increment is just beginning to form) to one (a complete increment has formed) as well as all values in between. When plotted as a function of month or season, the mean marginal increment should describe a sinusoidal cycle with a frequency of one year in true annuli (Lehodey & Grandperrin, 1996; Vilizzi & Walker, 1999). Edge analysis does not assign a state of completion to the marginal increment, but rather records its presence as either an opaque or translucent zone (van der Walt & Beckley, 1997; Labropoulou & Papaconstantinou, 2000). It is the change in relative frequency of each edge zone which is plotted across months or seasons, but as with MIA, the cycle frequency should equal one year in true annuli. In both MIA and edge analysis, a yearly cycle of formation can be difficult to distinguish from other frequencies, contributing to their poor performance as validation methods. Changes in the seasonal timing of the marginal increment with age or location undoubtedly contribute to the problem; significant and unexplained differences among years have also been noted (Pearson, 1996; Cappo et al., 2000). Despite the problems inherent in their use for age validation, both MIA and edge analysis are well suited for determining the month or season of formation of the opaque or translucent zone once annulus formation has been validated through independent means (Pearson, 1996; Natanson et al. 2001).
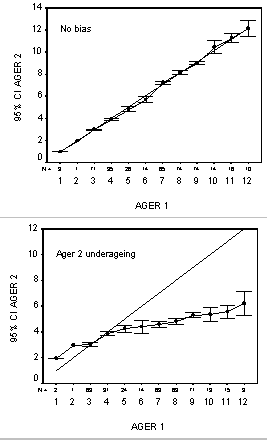
There are several reasons why MIA may provide misleading results. Prominent among these is the fact that the marginal increment is most easily discerned in young, fast-growing fish, a life history stage where the marginal increment may accurately confirm the formation of annual increments. The problem arises when the 'validation results' are later applied to older fish, contrary to the assumptions of all age validation methods. Many studies have reported age validation based on MIA of young fish, but noted that the same ageing structure and/or approach provided incorrect ages in older fish (Campana, 1984; Hyndes et al., 1992; Lowerre-Barbieri et al., 1994). More troublesome are the instances where age validation based on MIA of young fish later evolved to form the basis for routine ageing of the species across all age groups. For example, MIA of scales in young snapper (Pagrus auratus Bloch and Schneider) quickly evolved to become the basis for all scale ageing of the species in several countries; OTC mark-recapture results later showed that scale ages underestimated true age in older fish (Francis et al., 1992). A nearly identical situation took place in the northeast Pacific, where all routine ageing of sablefish (Anoplopoma fimbria Pallas) by several countries was based on scales validated with MIA. It wasn't until otolith OTC mark-recapture studies were completed that it was realized that scale ages were underestimating the age of older fish by up to a factor of four (McFarlane & Beamish, 1995). Note however that MIA misuse is not restricted to scale ageing. Annuli in whole otoliths of redfish (Sebastes spp) were validated using MIA, and subsequently became an accepted procedure of many organizations for ageing these long-lived fishes; subsequent validations have demonstrated that whole otoliths grossly underestimate age in older fish (Campana et al., 1990). The conclusion is clear: when proper age validation studies are lacking, researchers will often seize upon any available studies which can corroborate their age interpretations. And since MIA is one of the few validation methods which is restricted to young, fast-growing fish, it is also the most likely to lead to serious ageing error when applied blindly.
It is difficult to recommend the use of a technique where the data can be so subjectively interpreted. Nonetheless, the approach is valid if done with sufficient rigor. Four aspects of a rigorous protocol appear to be important: 1) samples must be completely randomized before examination, with no indication to the examiner when the sample was collected; 2) a minimum of two complete cycles needs to be examined, in accordance with accepted methods for detecting cycles; 3) the results must be interpreted objectively, extending well beyond the "looks like a cycle to me" interpretation that is so commonly used. It is difficult to recommend one statistical test that would apply in all circumstances, although a variety of useful options have been offered (Vilizzi & Walker, 1999; Cappo et al. 2000). At a minimum however, there should be significant differences among some or all of the seasonal groups in each of the cycles examined; and 4) the MIA should be restricted to only a few age groups at a time, ideally only one. As noted by Hyndes et al. (1992) in a study of whole otoliths, examination of a sample which includes young, annulus-producing fish and older, non-annulus producing fish, can easily result in a significant annual cycle for the sample as a whole, despite the fact that the older fish by themselves would not show such a cycle. In other words, the validation results should be considered to be age-specific.
Captive Rearing
Captive rearing is generally discounted as a reliable means of validating annulus formation, but maintains some utility at the daily level. Laboratory environments are seldom able to mimic natural environments, due to their artificial photoperiods, temperature cycles, feeding schedules and limited space for diurnal vertical migrations. Since annulus formation is strongly influenced by the environment (Schramm, 1989; Beckman & Wilson, 1995), an artificial environment is likely to produce artificial annuli. Daily growth increments are much less affected by environmental conditions, due to the endocrine-driven endogenous rhythm which controls their formation (Campana & Neilson, 1985). While laboratory environments are well known for resulting in daily increments of altered appearance, the frequency of their formation is not generally an issue unless the rate of growth is unnaturally low. For this reason, laboratory experiments to confirm daily increment formation of known-age or chemically-marked fish are common (Geffen, 1992).
Mesocosms, ocean pens and outside enclosures provide improved and more natural rearing environments for validation studies than do indoor locations. For otolith microstructure studies in particular, outdoor rearing can be expected to produce daily increments which are quasi-natural in appearance and frequency, although growth rates can be artificially high in hatchery operations (Campana & Neilson, 1982; Folkvord et al., 1997). At the annual level, outdoor rearing can also be expected to produce more natural-looking growth structures, although it has not yet been determined if annuli produced under such conditions are equivalent to those of wild fish (Schramm, 1989).
For more information on age validation methods, and for a review of methods which are used for age corroboration, please see Campana (2001).